
|
 |
|
{Page 77}
School of Chemical Engineering and Materials Science, The University of Oklahoma, Norman, Oklahoma
A generalized modified BWR equation of state developed from normal paraffin hydrocarbon data has been tested for prediction of the thermodynamic properties of eight halogenated hydrocarbons. Average absolute deviations of predicted saturated properties from tabulated values were 1.61% for vapor pressure, 2.49% for liquid density, 2.38% for vapor specific volume, 1.75 Btu/lb for liquid enthalpy, 1.21 Btu/lb for vapor enthalpy, and 0.05 Btu/lb - °R for both liquid and vapor entropy.
| Introduction | The GMBWR Equation | GMBWR Characterization Parameters for Halogenated Hydrocarbons | Comparison of Predicted and Reported Saturated Properties | Conclusions | References | Table of Contents | Home |
A generalized modified BWR equation of state (1) developed primarily for hydrocarbon thermodynamic properties predictions was tested in this study for prediction of halogenated hydrocarbon thermodynamic properties. The major impetus for this study was provided by needs in the geothermal power industry, although other industries also can utilize the results. The MBWR (Modified Benedict-Webb-Rubin) equation is presently being used in the geothermal power industry for convenient prediction of power cycle working fluid properties for hydrocarbons (2). Although the Martin-Hou equation has been used for halogenated hydrocarbons (3), difficulties in its use have been encountered (2, 4), probably because this equation was not originally intended for use in the compressed liquid region at low reduced temperatures. The present work should be considered to be a feasibility study of use of the MBWR equation for halogenated hydrocarbons. Only generalized equation of state parameters have been used herein. Obviously, determination of specific parameter values for each halogenated hydrocarbon would yield considerably improved predictions. The determination of specific MBWR parameters and/or development of an improved equation of state will be carried out in subsequent work.
| Introduction | The GMBWR Equation | GMBWR Characterization Parameters for Halogenated Hydrocarbons | Comparison of Predicted and Reported Saturated Properties | Conclusions | References | Table of Contents | Home |
The GMBWR (Generalized Modified Benedict-Webb-Rubin) equation of state, the related thermodynamic functions, and methodology for computations are well documented (1) and therefore, only a summary discussion of the GMBWR equation is presented here. The MBWR equation for the absolute pressure, P, is the following function of absolute temperature, T, and molar density, r ,
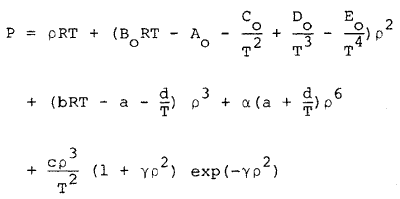 |
(1) |
where R is the universal gas constant and Bo, Ao, etc. are the eleven MBWR equation of state parameters. The GMBWR results when the following generalized relations are used for the MBWR parameters of a substance, where To , ro , and omega are, respectively, the critical temperature, critical density and acentric factor of the substance and Aj and Bj (j = 1, 2, . . . 11) are universal parameters given in Table 1.
{Page 78}
| (2) | |
 |
(3) |
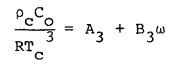 |
(4) |
 |
(5) |
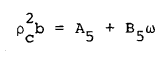 |
(6) |
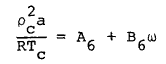 |
(7) |
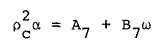 |
(8) |
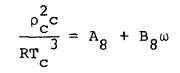 |
(9) |
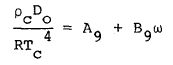 |
(10) |
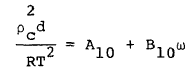 |
(11) |
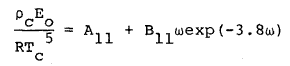 |
(12) |
Predictions of the thermodynamic properties of a given nonpolar substance (such as a hydrocarbon) using the GMBWR equation require only specification of the critical temperature, Tc, the critical density, rc, and the acentric factor w . For other substances, the parameter o can be treated as a pseudo acentric factor, that is, as a characterization parameter to be determined from experimental data such as vapor pressure data, rather than requiring that the value of o be determined from the defining relation for the acentric factor,
| (13) |
where Pro is the reduced vapor pressure (Po /Pc) and Tr is the reduced temperature (T/Tc).
| Introduction | The GMBWR Equation | GMBWR Characterization Parameters for Halogenated Hydrocarbons | Comparison of Predicted and Reported Saturated Properties | Conclusions | References | Table of Contents | Home |
The chemical formulas, molecular weights, critical constants, and boiling points of the twelve halogenated hydrocarbons considered in this study are given in Table 2. All property values used in this work were taken from ASHRAE tabulations (5). Given the values of the critical temperature and critical density in Table 2, the only task to allow use of the GMBWR equation for halogenated hydrocarbons was determination of the pseudo acentric factor for each substance.
To determine the optimal value of the pseudo acentric factor for a given fluid, an iterative search method was used in which the trial value of the pseudo acentric factor was varied until the average absolute deviation of predicted vapor pressures from reported values was minimized. Optimal values of the pseudo acentric factor were determined from vapor pressure values for three regions of temperature (1) 0° F and below, (2) 0° F and above, and (3) overall (total range of values). Figure 1 shows the behavior of the average absolute deviation of predicted from experimental vapor pressures for these temperature regions versus trial values of the pseudo acentric factor for sym.-dichlorotetrafluoroethane.
{Page 79}
Optimal values of the pseudo acentric factor determined in this manner are given in Table 3. For most of the fluids studied, the optimal value of the pseudo acentric factor is virtually independent of the temperature range of the vapor pressure data and is within the range of values of acentric factors reported in the literature (6, 7) and values obtained using estimation methods (6).
| Introduction | The GMBWR Equation | GMBWR Characterization Parameters for Halogenated Hydrocarbons | Comparison of Predicted and Reported Saturated Properties | Conclusions | References | Table of Contents | Home |
Evaluation of the capability of the GMBWR equation for predictions of the saturated thermodynamic properties of halogenated hydrocarbons was performed by comparing predicted and reported property values at the predicted vapor pressure at tabulated saturation temperatures. A summary of the results of these comparisons is given in Table 3. The results are very reasonable for most of the fluids studied. It can be noted in Table 3 that the overall average absolute deviations of predicted saturated properties from reported values for the eight halogenated hydrocarbons were 1.61% for vapor pressure, 2.49% for liquid density, 2.38% for vapor specific volume, 1.75 Btu/lb for liquid enthalpy, 1.21 Btu/lb for vapor enthalpy, and 0.05 Btu/lb–°R for both liquid and vapor entropy. Although these deviations are somewhat larger than for hydrocarbons (1), the GMBWR equation is sufficiently accurate for many engineering calculations involving these halogenated hydrocarbons.
| Introduction | The GMBWR Equation | GMBWR Characterization Parameters for Halogenated Hydrocarbons | Comparison of Predicted and Reported Saturated Properties | Conclusions | References | Table of Contents | Home |
The feasibility of using the GMBWR equation of state for prediction of the thermodynamic properties of halogenated hydrocarbons has been demonstrated. It was found that with the use of the three characterization parameters, critical temperature, critical density, and pseudo acentric factor, the saturated thermodynamic properties of eight halogenated hydrocarbons could be predicted with accuracies sufficient for many engineering calculations. The method used for determination of the pseudo acen-
{Page 80}
tric factor is simple and can be used for other halogenated hydrocarbons (with dipole moments less than 1.6 Debyes).
This study was supported by the Energy Research and Development Administration, Contract No. E(40-1)-5249.
| Introduction | The GMBWR Equation | GMBWR Characterization Parameters for Halogenated Hydrocarbons | Comparison of Predicted and Reported Saturated Properties | Conclusions | References | Table of Contents | Home |
1. K. E. STARLING, Fluid Thermodynamic Properties for Light Petroleum Systems, Gulf Publishing Co., Houston, 1973.
2. M. A. GREEN and H. S. PINES, "Calculation of Geothermal Power Plant Cycles Using Program GEOTHM", Lawrence Berkeley Laboratory Report LBL-3238 to ERDA (May 1975).
3. S. L. MILORA and J. S. TESTER, Geothermal Energy as a Source of Electric Power, Thermodynamic and Economic Design Criteria, MIT Press, Cambridge, Mass., 1976.
4. J. H. ESKESEN, "Study of Practical Cycles for Geothermal Power Plants", General Electric Co., Report to ERDA (Feb. 1976).
5. ASHRAE Thermodynamic Properties of Refrigerants, American Society of Heating, Refrigerating, and Airconditioning Engineers, Inc., 1969.
6. R. D. REID and T. K. SHERWOOD, The Properties of Gases and Liquids, McGraw-Hill, New York, 1966.
7. J. M. PRAUSNITZ, D. A. ECKERT, R. V. ORYE, and J. P. O'CONNELL, Computer Calculations for Multicomponent Vapor-Liquid Equilibria, Prentice Hall, Englewood Cliffs, 1967.